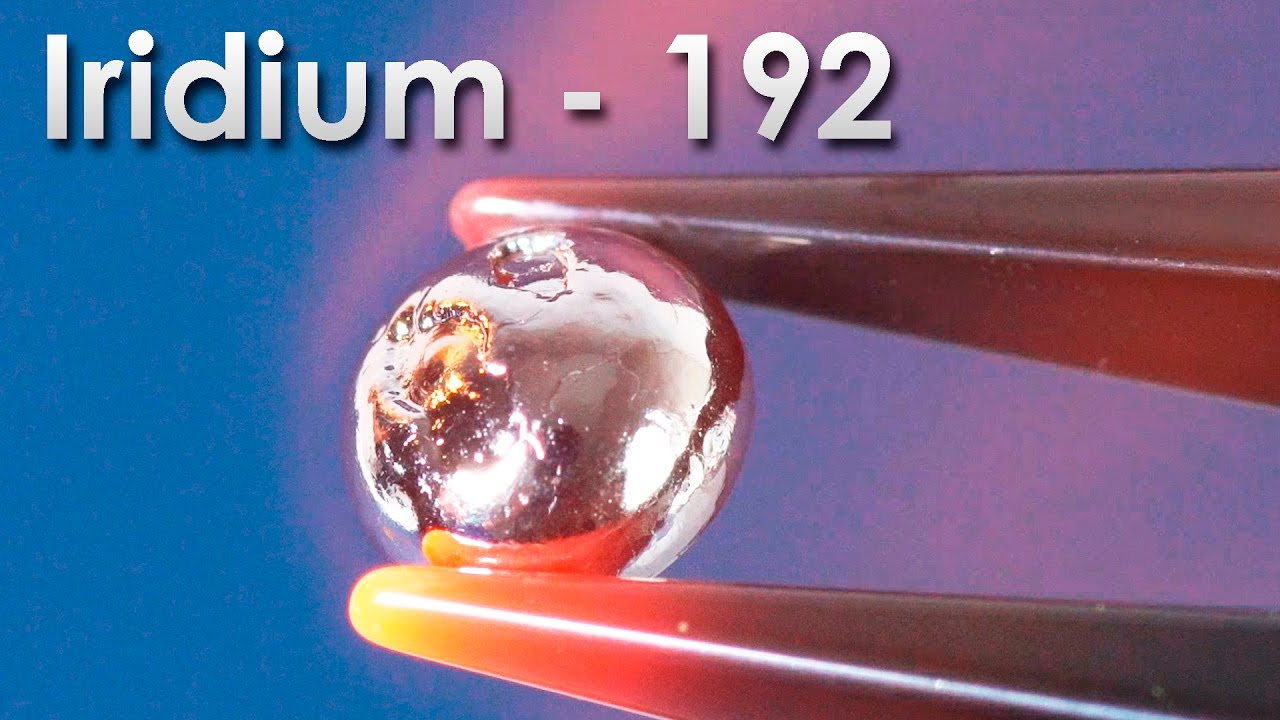
Erbium - A Metal, Which CREATES QUANTUM INTERNET!
Thanks for the erbium chloride: http://onyxmet.com/
Patreon: https://www.patreon.com/Thoisoi?ty=h
Facebook: https://www.facebook.com/thoisoi2
Instagram: https://www.instagram.com/thoisoi/
Do not repeat the experiments shown in this video!
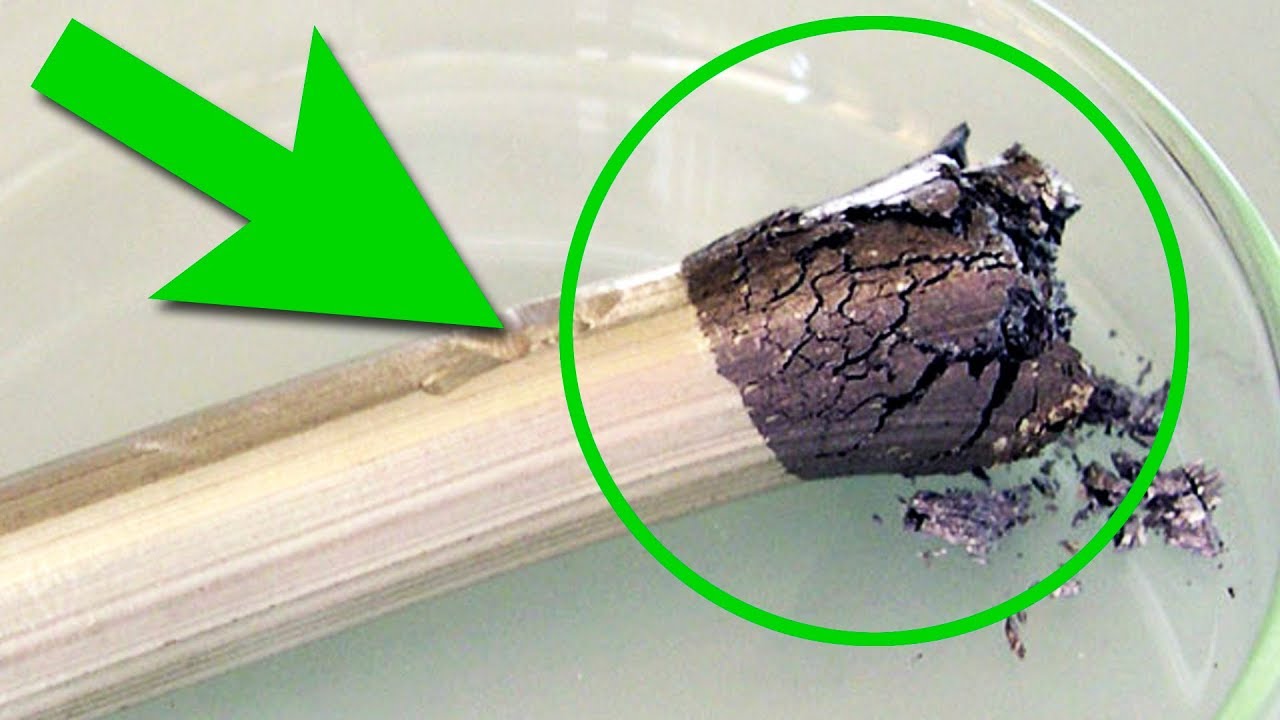
Today, I would like to tell you about one more unusual rare-earth metal - about erbium. Like all the other lanthanides, erbium belongs to group 6 in the periodic table. Its atomic number is 68. The history of this element is quite interesting. As many other rare-earth metals, erbium was discovered in Sweden but a small village named Ytterby that is located close to Stockholm was especially profound in this respect. From 15-th century on quartz for blacksmiths was mined in this village and from 18-th century on feldspar for potters was also mined here. In 1787 lieutenant Carl Arrhenius planned to build fortifications in this village but he accidentally saw an unusual black stone that he studied later on because he was was fond of chemistry but he didn’t find anything interesting in this stone. Later still Johan Gadolin, a Finish chemist, managed to find out that 38% of its content was an unknown element and along with that he named the mineral after himself naming it gadolinite. He named the new unknown element ytterbite after the settlement it was found in - Ytterby. Later in the middle of 19-th century another Swedish scientist whose name was Carl Mosander found out that ytterbite consisted of a mix of oxides of new metals. He named them erbium which was an oxide of erbium and terbium which understandably was an oxide of terbium. During the next 30 years the names of these two elements would change until finally in 1905 a pure oxide of erbium was extracted and all obscurities ended. The pure metal was first extracted only in 1934 through the reduction of erbium chloride using potassium vapor as the reducing agent. Nowadays, however, erbium is extracted from such a mineral as xenotime which is mostly found in China. The Chinese have monopolized the rare-earth metals market that is the reason why all the devices are made there, because making them requires rare-earth metals. Getting back to the point, the extracted metallic erbium is very similar to other lanthanides, to terbium for instance. Although it gets attracted to magnets much worse than terbium. By the way to check out the magnetic properties of many elements see the link down in the description. Erbium compounds are of distinctive bright pink color. Thanks to their colors they were discovered when gadolinite was studied. Erbium reacts with acids quite actively. It forms pink erbium chloride, which is slightly more pale than neodymium chloride, when submerged in hydrochloric acid. Like neodymium and holmium compounds, it changes color depending on lighting. In LED light it is more gray whereas in fluorescent lamp light it is bright pink. Chemical properties of all lanthanides are very similar and erbium is not an exception. When ground on our dear grinding wheel, it sparks beautifully burning down in the air and forming 3 valent erbium oxide. However, from the physical point of view erbium and its compounds are among the most widely used.