Thallium - The MOST TOXIC METAL ON EARTH!
Patreon: https://www.patreon.com/Thoisoi?ty=h
Facebook: https://www.facebook.com/thoisoi2
Instagram: https://www.instagram.com/thoisoi/
Do not repeat the experiments shown in this video!
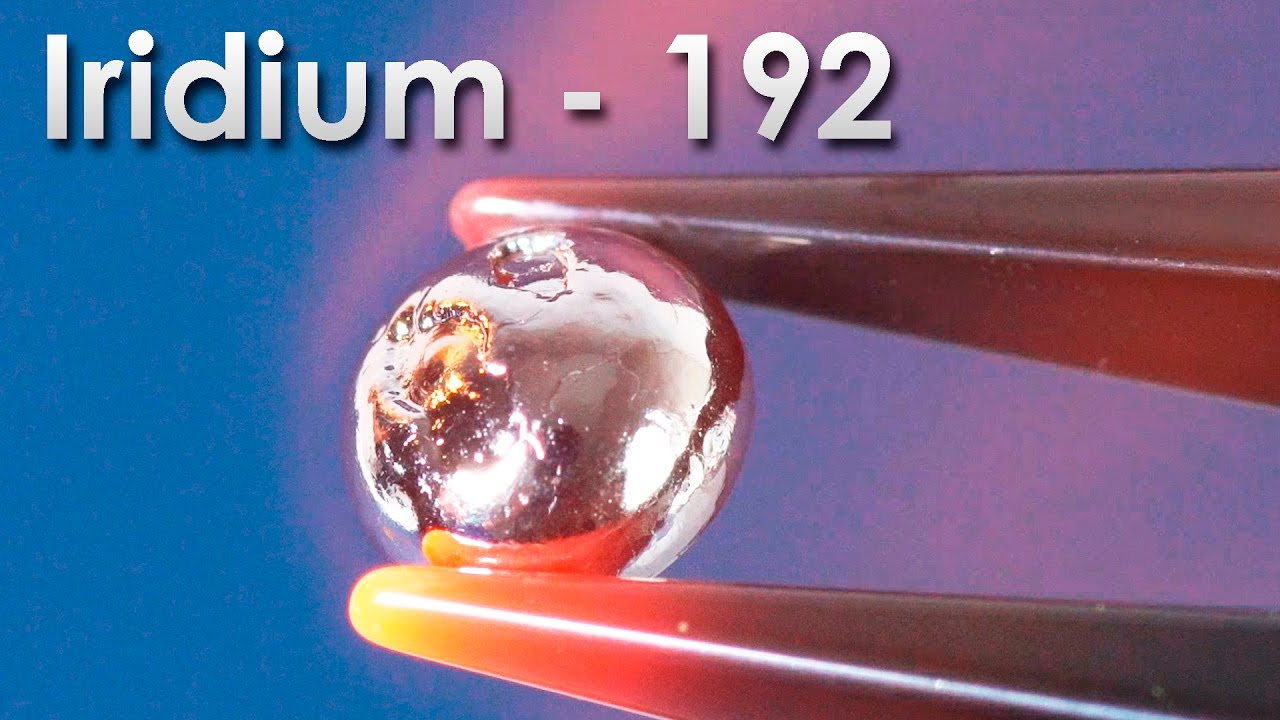
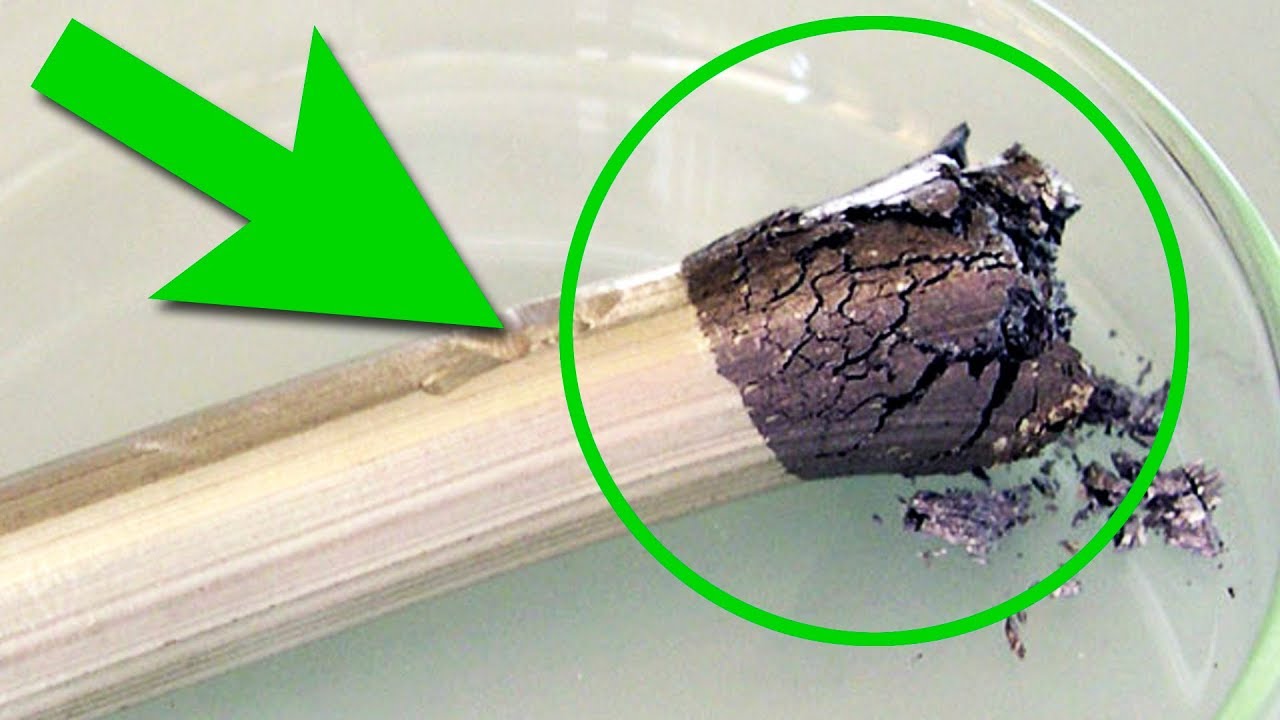
Today I will tell you about the most toxic metal on earth - about thallium. In the periodic table of chemical elements thallium lies on the bottom of group 13 having an atomic number 81. Let us start off with a little bit of history. Thallium was first discovered in 1861 by an English scientist William Crookes and also simultaneously by a French chemist Claude-Auguste Lamy. It was discovered thanks to the green colour of flames, that compounds of this metal would give. Thallium was discovered when scientists studied rocks containing lead. Nowadays, it is mostly extracted from sulfidic heavy metals, such as crookesite and “gicionite” if my rendering of their names is correct. I’ve got quite old pieces of thallium for my experiment that were produced back in 1970. Since then they have been strongly oxidized and covered in dark thallium oxide. Usually to protect thallium from getting oxidized it is stored in glycerol. Do not worry, we have taken all the necessary precautionary measures. Do not try this at home! To see the shiny surface of metallic thallium, I submerged my piece of thallium in concentrated nitric acid where it slowly began to dissolve forming nitrites of this metal. Thallium’s oxides have been washed away, the metal looks shiny with bluish shades. Without its oxides this metal can easily be confused with tin or other safe metals that is why thallium is quite treacherous. It can easily be melt down because its melting point is just 304 degrees Celsius. Molten thallium oxidizes very quickly when exposed to air covering in dark thallium oxide layer. This sets it apart from other metals belonging to group 13. For instance chemical activity of metals starting from aluminium and finishing with indium steadily decreases. Indium doesn’t even oxidise when it is exposed to air and remains shiny. Thallium, however, is more active and not only has +3 oxidation state as metals placed higher in the periodic table but it also has +1 oxidation state which is quite unusual. Thallium used to be considered alkali metal for some time after it was discovered. If an oxidized droplet of thallium is submerged in nitric acid, the oxide layer will immediately dissolve after that metal’s shiny surface can be seen. Thallium’s solidness is similar to that of lead. It is also quite soft and can easily be twisted. We also used to have thallium nitrate we could use for a few experiments in our laboratory. By the way thallium compounds are the most toxic among all metals because toxic arsenic, for instance, belongs to metalloids class but we will speak about toxicity a bit later. Having put off all the fears and switched the hood to maximum suction setting I continued running my experiment with this element. Thallium nitrate doesn’t dissolve in water well and looks like white crystals. Thallium compounds, thallium sulfate to be precise, had been widely used as rat poison until 1972 but later on the practice was abandoned, because it is too toxic and it became clear that thallium sulfate was toxic to people too. If you add potassium iodide to thallium nitrate solution, there will form beautiful yellow thallium iodide sediment. In spite of being toxic this chemical has a few applications.